

I am grateful to Professor Pekka Pyykkö (University of Helsinki, Finland) who provided the nuclear quadrupole moment data in this and the following two references. You might suppose that the mass spectrum would look. Where given, data for certain radioactive nuclei are from this reference. Chlorine has two isotopes, 35Cl and 37Cl, in the approximate ratio of 3 atoms of 35Cl to 1 atom of 37Cl. Mason in Multinuclear NMR, Plenum Press, New York, USA, 1987. It is a cosmogenic radioisotope of chlorine with a.

I am grateful to Professor Robin Harris (University of Durham, UK) who provided much of the NMR data, which are copyright 1996 IUPAC, adapted from his contribution contained within this reference. Chlorine 36 (Cl-36) is the chlorine isotope whose nucleus consists of 17 protons and 19 neutrons. 5, John Wiley & Sons, Chichester, UK, 1996. Yet, chlorine stable isotopes ( 37 Cl & 35 Cl) of dissolved chlorides are proved to be powerful tools to describe transport mechanism of. Harris in Encyclopedia of Nuclear Magnetic Resonance, D.M. Magnetogyric ratio, γ (10 7 rad T ‑1 s -1) Table of NMR-active nucleus propeties of chlorine Ĭommon reference compound: KCl/D 2O, 0.1 M. Kuchitsu in Quantities, Units and Symbols in Physical Chemistry, Blackwell Scientific Publications, Oxford, UK, 1988.
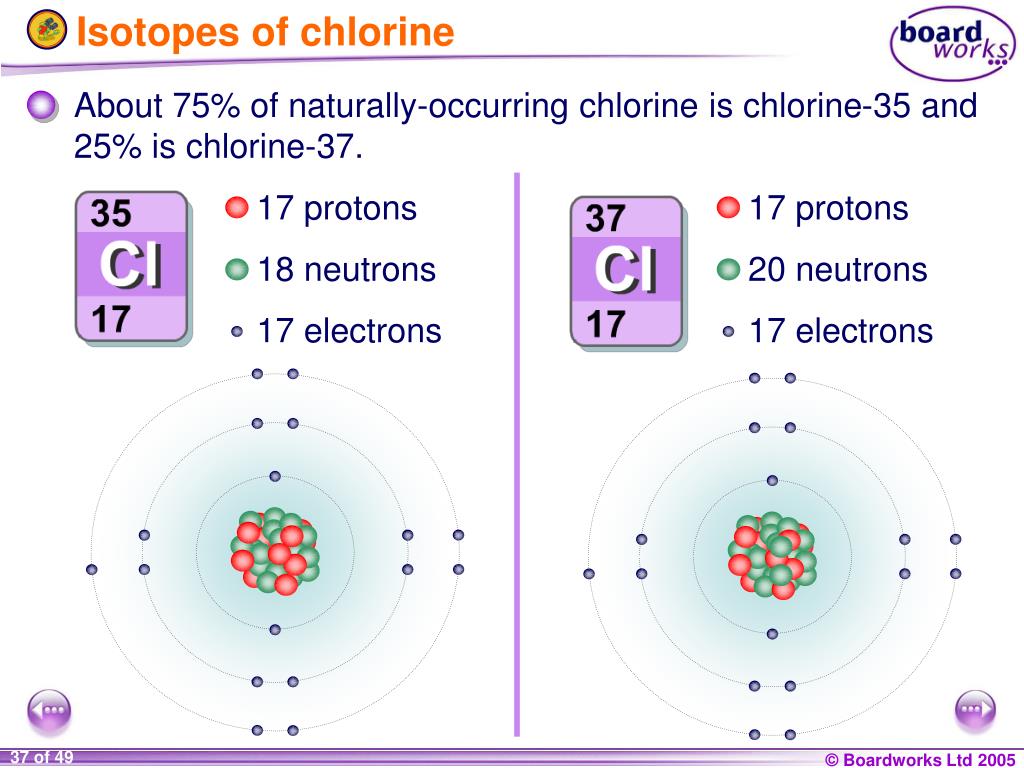
Pyykkö, personal communication, 1998, 204, 2008, 2010.Further data for naturally occuring isotopes of chlorine are listed above. Lide, (ed.), CRC Handbook of Chemistry and Physics 1999-2000 : A Ready-Reference Book of Chemical and Physical Data (CRC Handbook of Chemistry and Physics, CRC Press, Boca Raton, Florida, USA, 79th edition, 1998. This results from the relative abundance of 75.76 of chlorine-35 and 24.24 of chlorine-37. In the periodic table however, the mass of a chlorine atom is given as 35.45 u. I am grateful to Professor Pekka Pyykkö (University of Helsinki, Finland) who provided the nuclear quadrupole moment data in this and the following two references. It has two stable isotopes namely 35 Cl and 37 Cl. Where given, data for certain radioactive nuclei are from this reference. I am grateful to Professor Robin Harris (University of Durham, UK) who provided much of the NMR data, which are copyright 1996 IUPAC, adapted from his contribution contained within this reference. 5, John Wiley & Sons, Chichester, UK, 1996. Further data for naturally occuring isotopes of chlorine are listed above.


 0 kommentar(er)
0 kommentar(er)
